HUTCHMED: Going global to put cancer on trial
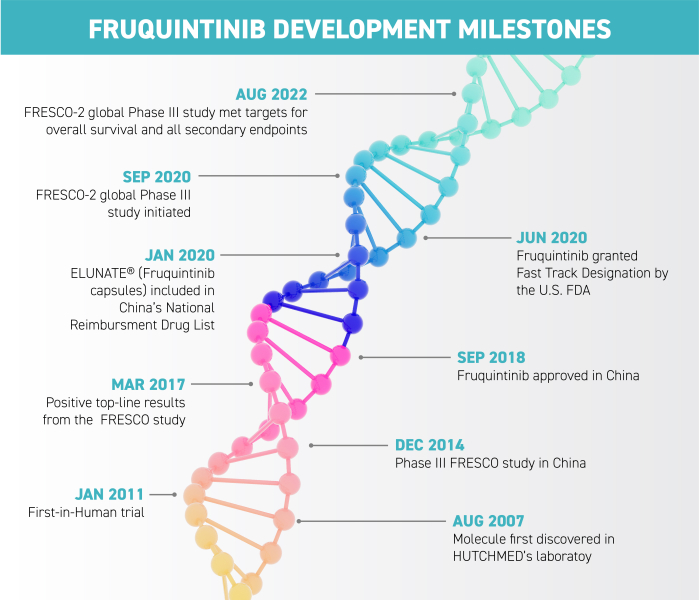
HUTCHMED’s journey with its potential blockbuster, fruquintinib, has seen it gain approval in Mainland China where it is already providing benefits to patients with metastatic colorectal cancer. A new globe-spanning study aims to bring its benefits to the rest of the world. But getting there, especially during the pandemic, hasn’t been easy.
Fruquintinib: Approved in China
The molecule in question, fruquintinib, was approved in China in 2018 after successfully completing the FRESCO Phase III trial there. In most jurisdictions, drugs must pass a Phase I (safe dosage) and Phase II (proving clinical efficacy) trial before going on to “the big one”, Phase III, where the efficacy and safety are proven in a large-scale clinical trial.
The new Phase III trial has its own name: FRESCO-2. This trial required a massive scientific, medical, operational and administrative effort demanding the collaboration of a myriad of stakeholders across continents and cultures.
In China, the drug has proved effective at improving overall survival (OS) – the primary endpoint of the original FRESCO study – and progression-free survival (PFS) – the length of time that patients live with the disease without tumour progression. The cancer under study, metastatic colorectal cancer, is the #2 biggest cancer killer in the US and #3 in Europe. For a cancer of this lethality and patients with very limited life expectancy, having a statistically significant and clinically meaningful overall survival benefit and progression-free survival is very important for patients and doctors. Benchmarking against other therapies, fruquintinib may stop the tumour from progressing for an additional 1.8 to 2.8 months. Fruquintinib demonstrated 9.3 months in median overall survival in China – a significant improvement on existing options. It effects a 35% reduction of risk of death and a 74% reduction in risk of progression, a highly meaningful clinical result. This patient population has very limited treatment options otherwise.

Going global
Armed with these data from China, HUTCHMED could move with confidence to the global study. The study was conducted in the US, Europe, Japan and Australia, encompassing a total of 14 countries. Testing a cancer drug is not as simple as giving it to patients with the disease to see if it works or not. Every country has its own approved “clinical practice” for the cancer in question.
HUTCHMED needed to work with all of its partners to ensure fruquintinib was being tested in a way that would allow it to be approved within a recognised clinical practice and regulatory framework. The design of the trial had to align with current best practice in such a way that it could also be measured against current options – or lack thereof. Failure to align trial design with known protocols would likely mean no approval as the outcome.
Regulators are just one of the key stakeholders that HUTCHMED works with. Doctors are also key partners. Researchers refer to them as “investigators” and “treating physicians” interchangeably to reflect their roles. They provide valuable input during the trial design phase and, of course, by communicating the potential risks and benefits to patients.
Working with regulators and investigators during COVID-19 posed its own problems. Regulatory agencies that were previously considered more bureaucratic and demanding of face-to-face meetings established new online protocols and, where possible, telemedicine reporting was implemented. As Dr Michael Shi, Head of R&D and Chief Medical Officer of HUTCHMED says, “Physicians cannot procrastinate. Cancer patients are desperately waiting.”


Finding patients, purging bias
Bringing each “site” (cancer treatment centre) on board to participate involves research, cost and time. So sites that will recruit the most patients have to be carefully selected. Too many unproductive sites wastes time and resources. But too few sites results in low enrolment that slows the introduction of the medicine to the market and patients.
To find patients, they must, in the words of Dr Shi, “go where the patients are”. This includes academic, community practice and national health service cancer clinics in addition to private health services, depending on the country and local health culture.
HUTCHMED navigated this process to enrol almost 700 patients in 15 months – an impressive feat under normal conditions, even more so during the COVID-19 pandemic. Different countries or regions may vary in the speed and rates of patient enrolment, due to factors including culture, patient population and local medical practice.
European countries usually have very high unmet medical needs and the interest in clinical trial participation is often higher as a result. In the FRESCO-2 study, Europe contributed more than 70% of the patients. Japan was more challenging. However, the researchers managed to recruit nearly 10% of the total patients so the Japanese population is well represented and meets the requirements of registration in this important market.
Physicians cannot procrastinate. Cancer patients are desperately waiting.
More patients can be convinced to join clinical trials if the number receiving the placebo is reduced to, say, one-third of patients (instead of 50%). The use of placebos in a double-blind study is just one method of reducing bias in the study. Even doctors and nurses don’t know who is getting the placebo. Doctors might think they can tell from observing side effects, but surprisingly, sometimes patients receiving a placebo can also exhibit side effects or even start to feel better. Careful protocols have to be enforced over hundreds of sites to ensure that meaningful results that satisfy regulators can be confirmed. “Stratification factors” are identified to take into account variations in patient conditions such as when they were diagnosed with the cancer before treatment started.
At the end of the trial, the “code is cracked” to show which patients received fruquintinib as part of the trial and who had placebos – and what the results are. This is the big moment when the efficacy of the drug is revealed.

Final results to the fast track
In August this year, the trial results were revealed: the FRESCO-2 study met its targets for overall survival and progression-free survival, showing statistically significant and clinically meaningful benefits, with a 34% reduction in risk of death and a 68% reduction in risk of progression. This puts the HUTCHMED team and this clinical data package on the path for regulatory submissions in key jurisdictions around the globe. Fruquintinib was granted Fast Track Designation by the US Food and Drug Administration (FDA). Submissions and regulatory reviews will be ongoing in late 2022 and 2023, with expectations for approvals in late 2023 and early to mid-2024.
This encouraging global trial is just the beginning. The mechanisms through which fruquintinib affects this cancer could be used for other cancers, on its own or in combination with other innovative agents, and research in that direction is already underway. The regulatory approval process is long and complex, but at the end is the prize of extending people’s lives, and uplifting patients and their families. That makes it all worth the huge effort that HUTCHMED and their collaborators put into bringing solutions to doctors and patients around the world.
At the end of the trial, the “code is cracked”.